
Date of publication:2024-06-30 22:08:13
DIRUI, as one of the leading in vitro diagnostic companies in China, believes in producing high quality product that can help streamline procedures, whilst saving time and money for laboratories of all sizes and budgets.
In the clinical laboratory, Quality Control (QC) refers to the process of detecting analytical errors to ensure both the reliability and accuracy of patient test results. Poor performance can result in misdiagnosis, delayed/inappropriate treatment, increased costs and may even be potentially life threatening for the patient. External Quality Assessment (EQA) provides a means of assessing the analytical performance of a laboratory compared to other laboratories utilizing the same methods and instruments. Therefore, it is essential to conduct EQA for all laboratories to assure result accuracy.

RIQAS (Randox International Quality Assessment Scheme) is the world's largest international EQA scheme, with participation from over 76,200 laboratories in 145 countries. RIQAS offers laboratories a comprehensive and cost-effective EQA solution that helps meet regulatory requirements and ensures the reliability and accuracy of patient test results.
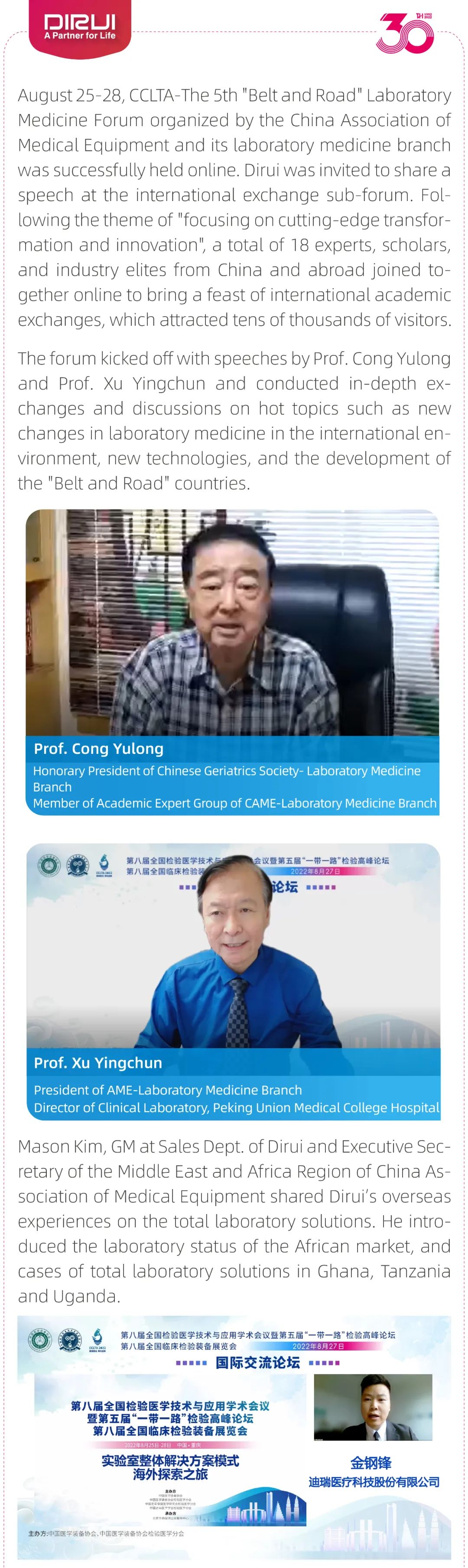
To further expand the knowledge and understanding of EQA and QC practices, in June 2024, DIRUI organized a webinar on the topic of "Interpretation and Application of RIQAS Report."
Randox manager Stephen Doherty was invited to present on the importance of EQA in the laboratories and to provide laboratories some great advice on EQA to improve DIRUI’s laboratory testing. This "solution-oriented" webinar attracted a large audience of DIRUI, DIRUI’s global partners, distributors and KOL experts by providing valuable insights.
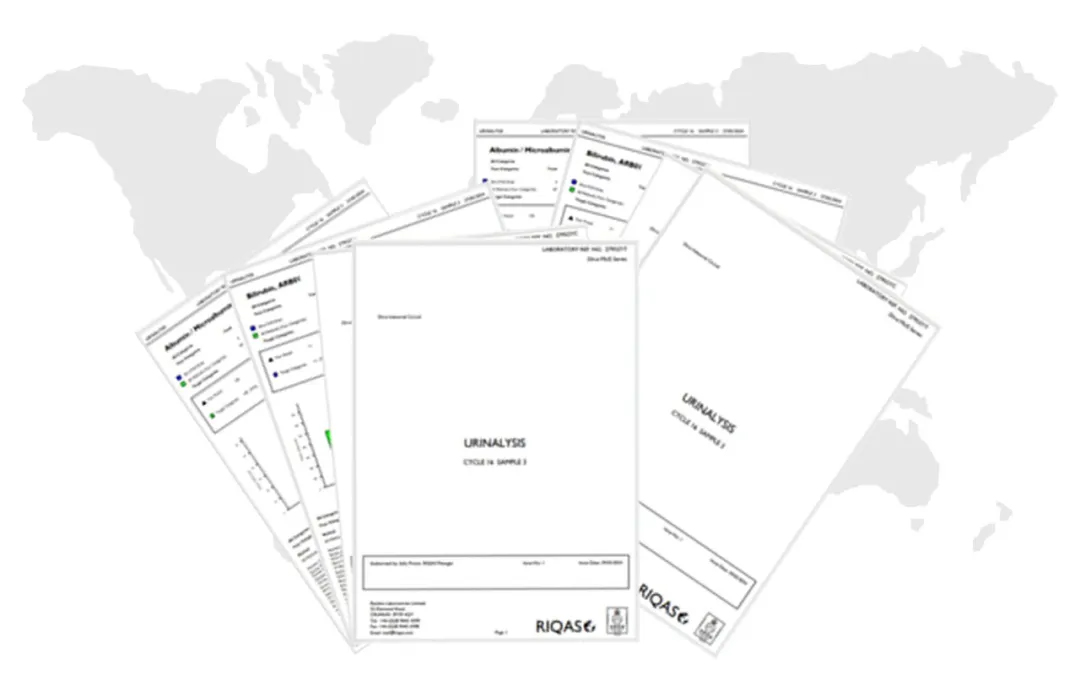 RIQAS Report
RIQAS Report
During the webinar, participants engaged in in-depth discussions with Stephen, addressing real-life scenarios and solutions encountered during use. Stephen's clear and insightful responses provided participants with valuable experience and new ideas for their future work.
The successful hosting of this webinar provided participants with a wealth of knowledge and experience sharing, further solidifying DIRUI's customer-centric philosophy and focus on product quality.
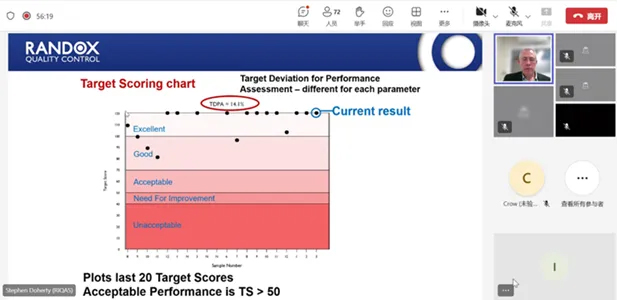
By collaborating with more internationally renowned institutions, DIRUI will continue to strive to improve our product quality and ensure that our customers receive the highest quality service and the most reliable test results. We look forward to future cooperation and co-innovation to make greater contributions to the global IVD industry.
 Overseas Engineers’ Training at DIRUI Headquarter (July 2024)
Overseas Engineers’ Training at DIRUI Headquarter (July 2024)
 A silent killer—Diabetic Kidney Disease
A silent killer—Diabetic Kidney Disease
 Lending a hand to the families affected by the Turkey earthquake
Lending a hand to the families affected by the Turkey earthquake
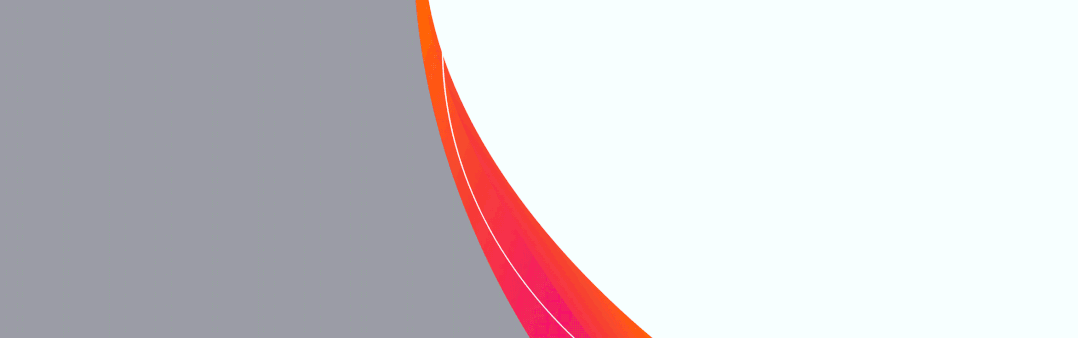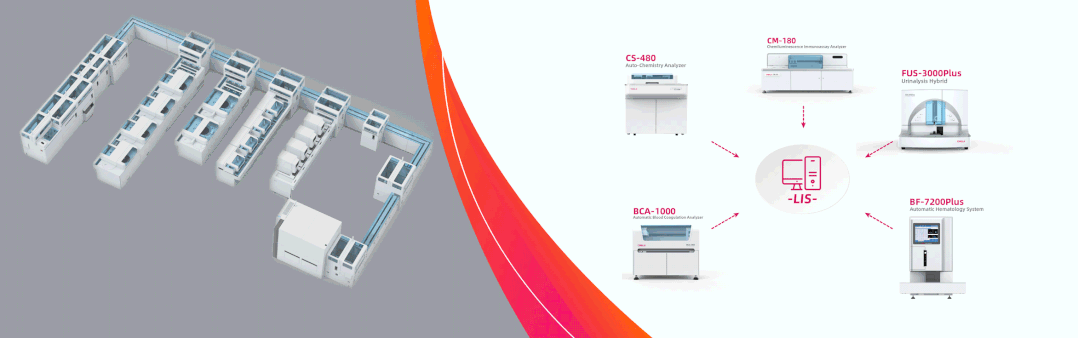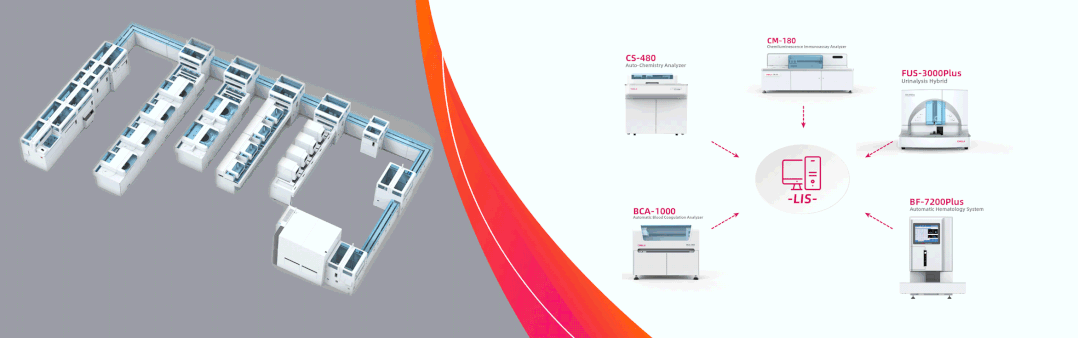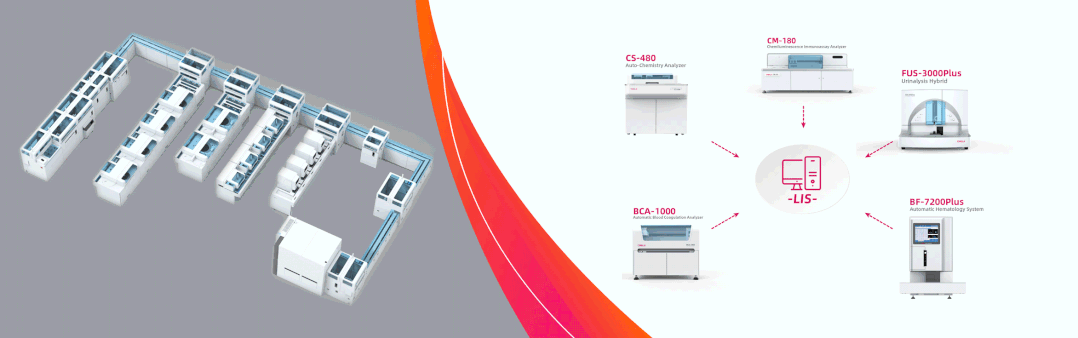 Dirui showcases complete solutions in Dubai
Dirui showcases complete solutions in Dubai
 DIRUI laboratory solutions showcase at ACBICON 2022
DIRUI laboratory solutions showcase at ACBICON 2022
 MEDICA sees NEW laboratory solutions from DIRUI
MEDICA sees NEW laboratory solutions from DIRUI
 2022 DIRUI CIS Region Laboratory Solutions Conference
2022 DIRUI CIS Region Laboratory Solutions Conference
 Total Laboratory Solutions were shared at CCLTA2022
Total Laboratory Solutions were shared at CCLTA2022
 DIRUI Blossom, AACC 2022
DIRUI Blossom, AACC 2022
 Diplomatic Delegation Visits DIRUI
Diplomatic Delegation Visits DIRUI
Office address: 3333 Yiju Road, New&High Tech. Development Zone, Changchun, Jilin 130103, P.R.
China
Tel: +86 431 85083742
E-mail: dirui@dirui.com.cn
DOWNLOAD CONTACT US CAREERS SITE MAP CEO EMAIL: drceo@dirui.com.cn
Copyright © 2010-2019 Dirui. All Rights Reserved.
Aftersales service hotline
400 808 7597