
Date of publication:2024-06-16 21:52:09
Being IVDR-compliant, DIRUI focuses on the creation and maintenance of a robust QMS throughout the manufacturing process while ensuring the post market surveillance. With the aim of supplying safe, reliable products and service to the customers around the globe, DIRUI rapidly responds to the prevailing regulations and works tirelessly to meet the conformity by obtaining the CE mark in accordance with the IVDR. As a company committed to lab diagnostic innovations, DIRUI, is dedicated to improving healthcare outcomes by creating better solutions that can meet the highest standards of quality, safety, and regulatory compliance.

 Overseas Engineers’ Training at DIRUI Headquarter (July 2024)
Overseas Engineers’ Training at DIRUI Headquarter (July 2024)
 A silent killer—Diabetic Kidney Disease
A silent killer—Diabetic Kidney Disease
 Lending a hand to the families affected by the Turkey earthquake
Lending a hand to the families affected by the Turkey earthquake
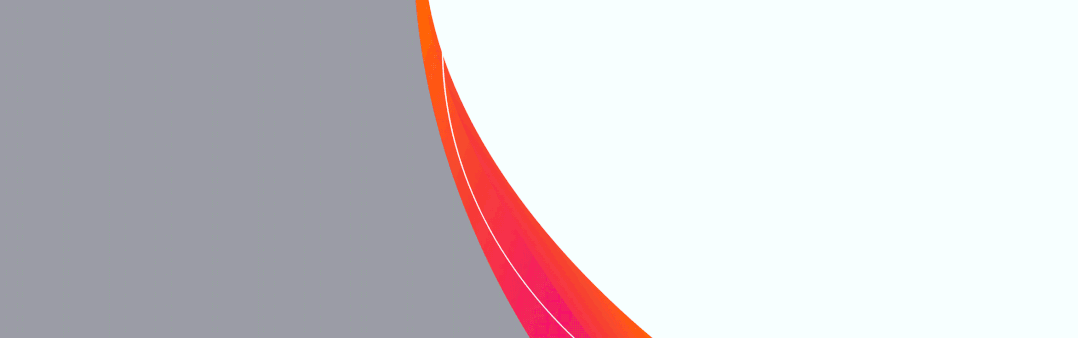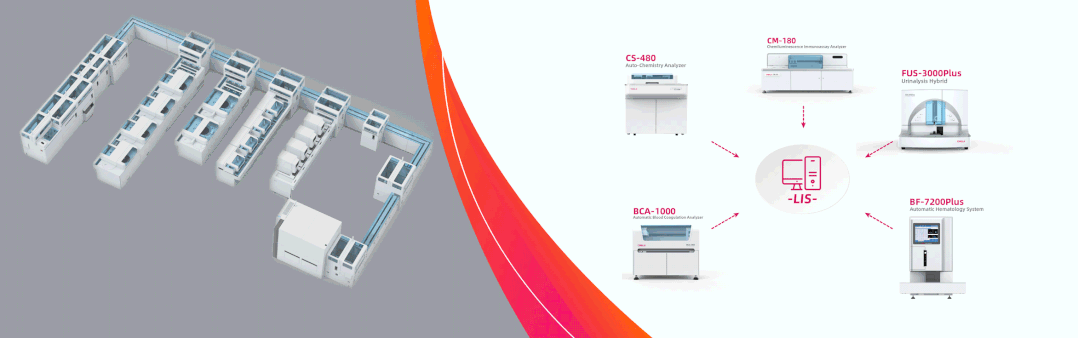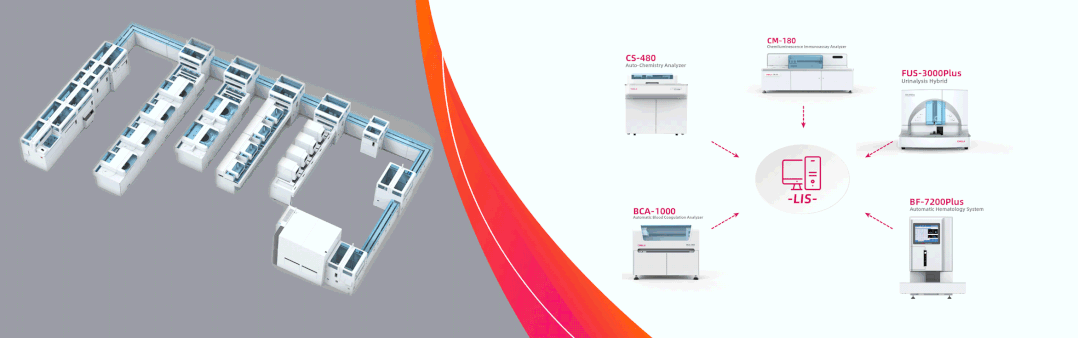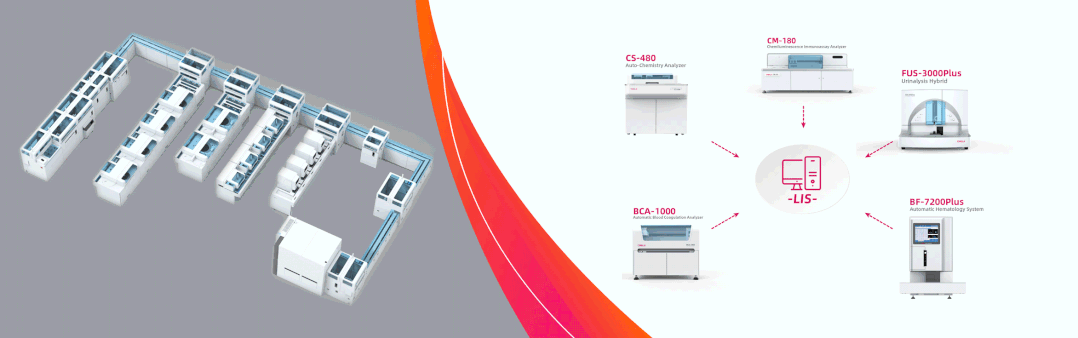 Dirui showcases complete solutions in Dubai
Dirui showcases complete solutions in Dubai
 DIRUI laboratory solutions showcase at ACBICON 2022
DIRUI laboratory solutions showcase at ACBICON 2022
 MEDICA sees NEW laboratory solutions from DIRUI
MEDICA sees NEW laboratory solutions from DIRUI
 2022 DIRUI CIS Region Laboratory Solutions Conference
2022 DIRUI CIS Region Laboratory Solutions Conference
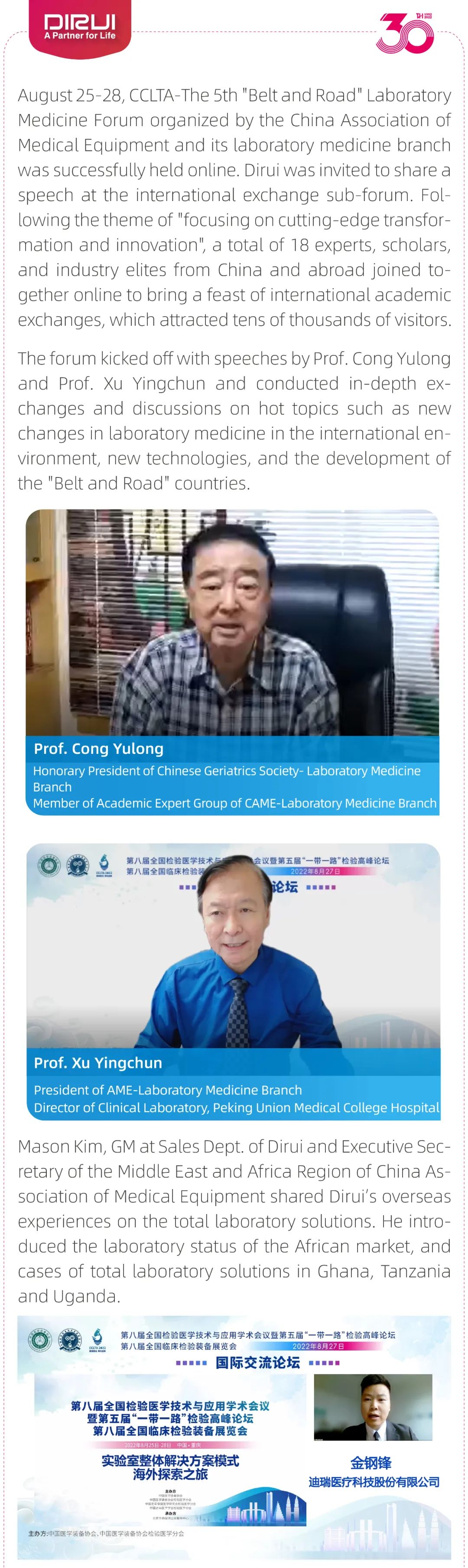 Total Laboratory Solutions were shared at CCLTA2022
Total Laboratory Solutions were shared at CCLTA2022
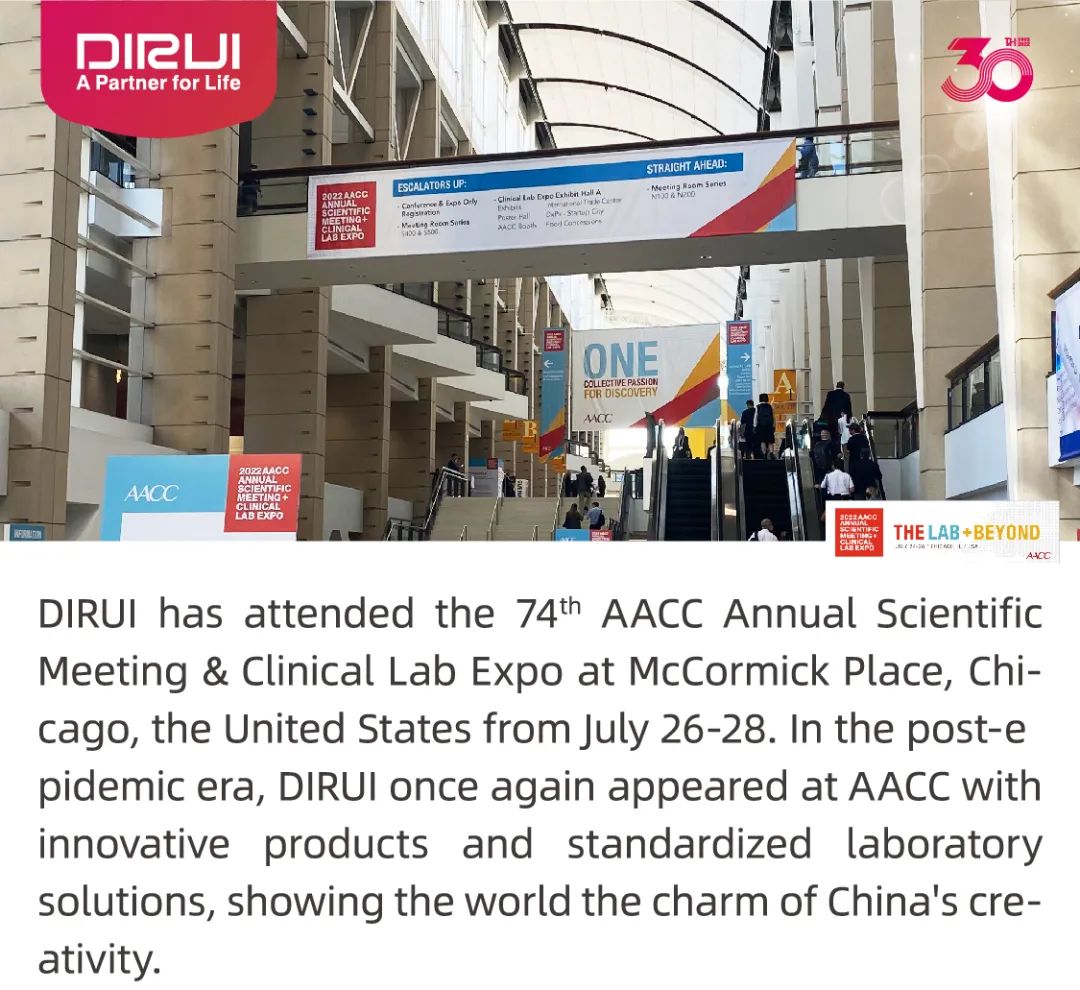 DIRUI Blossom, AACC 2022
DIRUI Blossom, AACC 2022
 Diplomatic Delegation Visits DIRUI
Diplomatic Delegation Visits DIRUI
Office address: 3333 Yiju Road, New&High Tech. Development Zone, Changchun, Jilin 130103, P.R.
China
Tel: +86 431 85083742
E-mail: dirui@dirui.com.cn
DOWNLOAD CONTACT US CAREERS SITE MAP CEO EMAIL: drceo@dirui.com.cn
Copyright © 2010-2019 Dirui. All Rights Reserved.
Aftersales service hotline
400 808 7597